oxygen electron configuration|8.3: Electron Configurations : Tuguegarao In order to write the Argon electron configuration we first need to know the . MTA bus Q65: map, schedule, stops and alerts. The bus operates between Jamaica and Flushing and serves 106 stops which are listed below. Bus Q65 schedule: services at this time. DESTINATION COLLEGE PT 110 ST via 164 ST via COLLEGE PT BL; at stop COLLEGE POINT BLVD/NYPD ACADEMY ENTRANCE;
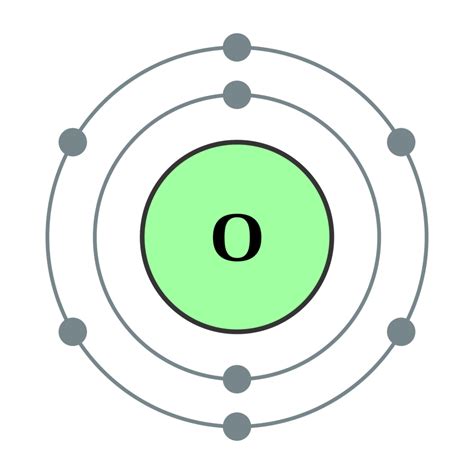
oxygen electron configuration,Learn how to write the electron configuration for oxygen using the period table or an electron configuration chart. See the video and the step-by-step tutorial for writing the electron configurations.
In order to write the Sulfur electron configuration we first need to know the .

How to Write the Electron Configuration for Carbon. Carbon is the sixth element with .How to Write the Electron Configuration for Neon. Neon is the tenth element with a .In order to write the Argon electron configuration we first need to know the .Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s .
How to Write the Electron Configuration for Nitrogen (N) Nitrogen is the seventh .Learn how to write the electron configuration for oxygen and its oxide ion using the Bohr model and the orbital model. Find out the group, period, block, valency, an.

For example, we know that Oxygen always forms 2- ions when it makes an ion. This would add 2 electrons to its normal configuration making the new configuration: O 2-1s 2 2s 2 .Hun 27, 2024 — Find the electron configuration of any element using this tool. Learn the rules, notation, and examples of electron configuration and valence electrons.oxygen electron configurationNob 14, 2013 — A step-by-step description of how to write the electron configuration for Oxygen (O). In order to write the O electron configuration we first need to know t.Ene 21, 2021 — What is the Electron Configuration of O. The distribution of electrons of an atom or molecule in atomic or molecular orbitals is called the electron configuration. For example, the electron configuration of the .Learn how to represent the arrangement of electrons in orbitals of atoms using the periodic table and rules such as Pauli exclusion principle and Hund's rule. See examples of oxygen and nitrogen electron .Ago 14, 2020 — Learn how to derive the predicted ground-state electron configurations of atoms using the Aufbau principle and the Pauli exclusion principle. See examples of orbital diagrams for atoms in the first and .
Dis 7, 2021 — Learn how to write the electron configuration of oxygen in standard and noble gas notation. This video is part of a playlist on organic chemistry by StudySession, a .
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that .At oxygen, with Z = 8 and eight electrons, we have no choice. One electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and a 1s 2 2s 2 2p 4 electron configuration. . The electron configurations of the elements are presented in Figure \(\PageIndex{2}\), which lists the orbitals in the order in .
oxygen electron configuration 8.3: Electron ConfigurationsNob 14, 2013 — A step-by-step description of how to write the electron configuration for Oxygen (O). In order to write the O electron configuration we first need to know t.
The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal .Abr 22, 2021 — Consider also the electron configuration of oxygen. Oxygen has 8 electrons. The electron configuration can be written as 1s 2 2s 2 2p 4. To draw the orbital diagram, begin with the following observations: the first two electrons will pair up in the 1s orbital; the next two electrons will pair up in the 2s orbital.Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right. . Oxygen accounts for about 23% of the atmosphere's mass with pairs of oxygen atoms stuck together to make .Dis 7, 2021 — Let's find the electron configuration of Oxygen! A single oxygen atom has 8 protons and 8 electrons, but how do we know where Oxygen puts its electrons, in w.Hun 14, 2015 — The electron configuration shows the distribution of electrons into subshells. This list of electron configurations of elements contains all the elements in increasing order of atomic number.. To save room, the configurations are in noble gas shorthand.This means part of the electron configuration has been replaced with the .
If we look at the element after nitrogen in the same period, oxygen (Z = 8) its electron configuration is: 1s 2 2s 2 2p 4 (for an atom). Oxygen has one more electron than nitrogen and as the orbitals are all half filled the electron must pair up. Occupation of .8.3: Electron ConfigurationsHun 27, 2024 — To find the electron configuration of oxygen: Look at the periodic table and find an atomic number of oxygen, which is 8. Fill these 8 electrons in the following order: 1s, 2s, and then 2p. Write the complete electron configuration of oxygen: 1s²2s²2p⁴. Identify the noble gas before oxygen, helium, and write using shorthand .
Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. [1] For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and .
Nob 27, 2021 — To write the orbital diagram for the Oxygen atom (O) first we need to write the electron configuration for just O. To do that we need to find the number of .However, molecular orbital theory describes the electronic configuration of molecular species such as oxygen molecule. The electronic configuration for a molecule containing 16 or more than 16 electrons according to MOT is given by: σ 1 s, σ * 1 s, σ 2 s, σ * 2 s, σ 2 p z, π 2 p x = π 2 p y, π * 2 p x = π * 2 p y, σ * 2 p z .
Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.Ene 21, 2021 — Oxygen Electron Configuration: O is an odourless, colorless, reactive gas. Its atomic number is 8 and it the life-supporting component of the air. It forms almost 20 percent of the earth’s atmosphere. It is also the most abundant element in the earth’s crust which is found mainly in the form of silicates, oxides, and carbonates.
Set 12, 2022 — We expect the two electrons that occupy these two degenerate orbitals to be unpaired, and this molecular electronic configuration for O 2 is in accord with the fact that the oxygen molecule has two unpaired electrons ( Figure \(\PageIndex{10}\)). The presence of two unpaired electrons has proved to be difficult to explain using Lewis .Okt 28, 2023 — For example, oxygen has the electron configuration 1s^2, 2s^2, 2p^4 which means it has six valence electrons in its outermost energy level. These valence electrons control how reactive oxygen is and how easily it .Electron configuration of Oxygen is [He] 2s2 2p4. Possible oxidation states are -2 . Oxygen is too chemically reactive to remain a free element in air without being continuously replenished by the photosynthetic action of living organisms.Nob 26, 2022 — Oxygen is placed in the 2 nd-period and group -16 in the periodic table based on its electron configuration.Let us explore different facts on its electron configuration. The electron configuration of Oxygen (O) will be 1s 2 2s 2 2p 4.Oxygen is a nonmetal gaseous substance that is also known as a p-block element.
oxygen electron configuration|8.3: Electron Configurations
PH0 · Oxygen Electron Configuration (O) with Orbital Diagram
PH1 · Oxygen Electron Configuration
PH2 · How to Write the Electron Configuration for Oxygen and O2
PH3 · Electron Configurations
PH4 · Electron Configuration for Oxygen (O)
PH5 · Electron Configuration Calculator
PH6 · 8.3: Electron Configurations
PH7 · 6.4 Electronic Structure of Atoms (Electron Configurations)
PH8 · 2.4 Electron Configurations